

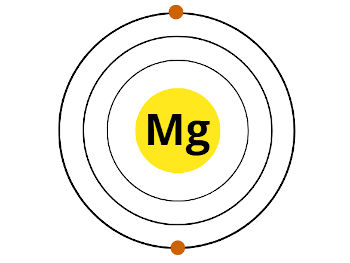
HCP (Hexagonal close packing) Melting point 923 K or 650 ☌ or 1202 ☏ Boiling point 1363 K or 1091 ☌ or 1994 ☏ Density 1.74 g/cm 3 Main isotope 24Mg Who discovered Magnesium and when?īefore knowing this reason, first of all I want to ask you a simple question. Protons 12 Neutrons 12 Electrons 12 Symbol Mg Atomic massĢ, 8, 2 Electronic configuration 3s 2 Atomic radiusġ73 picometers (van der Waals radius) Valence electronsĢ 1st Ionization energy 7.646 eV Electronegativity Shiny grey State (at STP) Solid Position in Periodic table Let’s dive right into it! Magnesium Element (Mg) Information Appearance So if you want to know anything about Magnesium element, then this guide is for you. In fact, the table mentioned below is the perfect information box (Which gives you every single detail about the Magnesium element in Periodic table.) The electronic configuration of Magnesium will be 1s2 2s2 2p6 3s2.This is a simple and easy guide on Magnesium element. How do you write the electron configuration for Magnesium? The electronic configuration of Magnesium will be 1s2 2s2 2p6 3s2. What is the electronic configuration of Magnesium 12? What is the boiling Point of Magnesium in Kelvin?īoiling Point of Magnesium in Kelvin is 1363 K. Melting Point of Magnesium in Kelvin is 923 K. What is the melting Point of Magnesium in Kelvin?
What is the boiling Point of Magnesium?īoiling Point of Magnesium is 1363 K.

Magnesium has 12 electrons out of which 2 valence electrons are present in the 3s2 outer orbitals of atom. How many valence electrons does a Magnesium atom have? The element Magnesium was discovered by J. It is located in group 2 and period 3 in the modern periodic table. Magnesium is the 12 element on the periodic table. Magnesium is a chemical element with the symbol Mg and atomic number 12. What is the position of Magnesium in the Periodic Table? Magnesium is a chemical element with symbol Mg and atomic number 12. To form abbreviated notation of electronic configuration, the completely filled subshells are replaced by the noble gas of the preceding period in square brackets. The abbreviated electronic configuration of Magnesium is 3s2. What is the abbreviated electronic configuration of Magnesium? The electronic configuration of Magnesium is 1s2 2s2 2p6 3s2.
What is the electronic configuration of Magnesium? Magnesium Thermal Properties - Enthalpies and thermodynamics Optical Properties of Magnesium Refractive IndexĪcoustic Properties of Magnesium Speed of Sound Magnesium Magnetic Properties Magnetic Type Magnesium Heat and Conduction Properties Thermal Conductivity Refer to table below for the Electrical properties ofMagnesium Electrical Conductivity Hardness of Magnesium - Tests to Measure of Hardness of Element Mohs Hardness Refer to below table for Magnesium Physical Properties Densityġ.738 g/cm3(when liquid at m.p density is $1.584 g/cm3)


 0 kommentar(er)
0 kommentar(er)
